Hook & thesis
ImmunityBio is trading as a story stock right now: a clearer resubmission path for ANKTIVA and accelerating product revenue have re-priced expectations, but the company remains a binary bet on regulatory execution and commercial traction. If ANKTIVA stays on a plausible path to approval and early revenue momentum continues, shares have room to move higher from today's $6.08. If those elements stall, downside is large and fast.
My trade idea is a mid-term swing long focused on the regulatory cadence and upcoming clinical updates. The plan assumes the market continues to reward visible progress - not full approval - and that short sellers and momentum traders remain a tailwind as the story unfolds. That makes this a potentially asymmetric trade if you size it small and use a strict stop.
What ImmunityBio does and why the market should care
ImmunityBio is a clinical-stage immunotherapy company developing treatments that combine innate and adaptive immune activation to treat cancers and infectious diseases. Its headline asset for the market currently is ANKTIVA, a therapy focused on BCG-unresponsive papillary bladder cancer, where recent regulatory dialogue has opened a resubmission path. That regulatory clarity is the immediate fundamental driver for the stock.
Beyond ANKTIVA, the company is expanding its pipeline - including a Phase 2 CAR-NK program combined with an IL-15 superagonist - and is reporting strong product revenue growth that has already drawn bullish headlines. In short: ImmunityBio’s valuation is being priced off a therapy that can convert into meaningful revenue quickly if regulators and payors cooperate.
Hard numbers that matter
| Metric | Value |
|---|---|
| Current price | $6.08 |
| Market cap | $5.92B |
| Enterprise value | $6.19B |
| Price-to-sales | 71.82x |
| EV / Sales | 74.92x |
| EPS (TTM) | -$0.35 |
| Free cash flow | -$324,756,000 |
| Cash metric | $1.05 |
| Shares outstanding | 984,965,000 |
| Float | 330,570,998 |
| 52-week range | $1.83 - $8.28 |
These numbers tell the story: the market is valuing ImmunityBio as a company with meaningful near-term product revenue potential. Yet valuation multiples (P/S ~72x, EV/S ~75x) are extreme and imply the market expects substantial revenue growth to materialize. At the same time, the company is generating negative free cash flow (-$324.8M), has nearly a billion shares outstanding and remains dependent on regulatory outcomes and execution.
Why now? Catalysts that can move the stock
- Regulatory push - The FDA provided a resubmission pathway for ANKTIVA in bladder cancer earlier in January (01/20/2026). Continued progress and constructive feedback from the agency will be the primary catalyst for upside.
- Commercial traction - Management has reported sharp revenue growth recently and the market has responded. Next quarterly revenue print and cadence on product rollout will be watched closely.
- Clinical readouts - Early updates from the Phase 2 CAR-NK plus IL-15 superagonist program could add to the story if they show tolerability and signals of efficacy.
- Short interest dynamics - Short interest remains material (135M shares as of 01/30/2026) and daily short-volume has been elevated. Rapid downside or upside can be exaggerated by short covering or aggressive shorting.
Trade plan (actionable)
Thesis: Buy into continued regulatory progress and revenue momentum while protecting capital against a binary adverse outcome.
Primary trade (swing):
- Trade direction: long
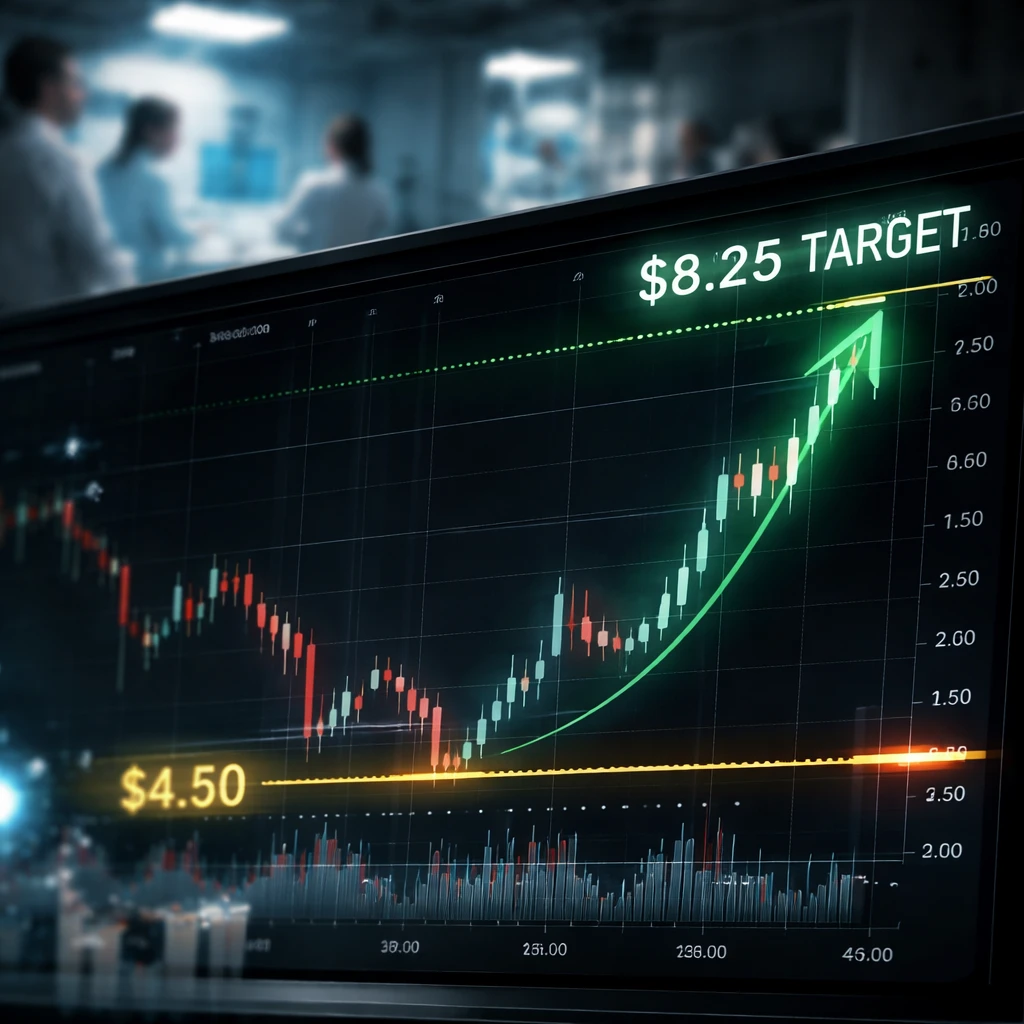
- Entry price: $6.08
- Stop loss: $4.50
- Target price: $8.25
- Time horizon: mid term (45 trading days) - this gives the market time to digest regulatory correspondence, the next revenue release and any early clinical updates.
Why these levels? Entry at $6.08 captures current momentum following regulatory clarity. The target of $8.25 is just below the 52-week high ($8.28) and represents a realistic upside if the resubmission path remains constructive and revenue beats continue to compound sentiment. The $4.50 stop limits downside to a controlled level if regulatory feedback or a negative revenue surprise reverses the momentum - that level also sits below recent moving averages and would signal a failure of the momentum trade.
Sizing and risk management
This is a high-risk trade. Because free cash flow is negative, shares outstanding are large and legal and regulatory outcomes are binary, position sizing should be small relative to portfolio risk tolerance - consider sizing at single-digit percent of speculative allocation. Use the stop without hesitation; the market can move quickly on headline news and short-volume dynamics can amplify moves.
Valuation framing
At roughly $5.9B market cap and $6.2B enterprise value the market is effectively pricing ImmunityBio as a revenue-generating growth company that will scale quickly. EV/Sales near 75x and P/S north of 70x are not typical biotech multiples unless revenue is growing extremely quickly and near-term profitability is plausible. The market appears to be banking on ANKTIVA converting to a commercial product and on the firm's product revenue to accelerate from recent triple-digit growth headlines.
That means the stock is priced for success. Any delay, material additional capitalization need, or disappointing clinical/regulatory news could force a large repricing lower. Conversely, a clean regulatory run and continued revenue acceleration could justify a higher valuation, at least temporarily, while markets attach a premium to visible sales growth.
Catalyst calendar to watch (next 45 trading days)
- Any new FDA correspondence on ANKTIVA or formal resubmission dates - primary catalyst.
- Quarterly revenue release and management commentary on product rollout.
- Interim clinical updates from the CAR-NK program or other pipeline assets.
- Short interest reports and daily short-volume spikes - these can amplify price action and create squeeze dynamics.
Risks and counterarguments
- Regulatory risk - Approval is not guaranteed. The resubmission path reduces uncertainty but the FDA can still demand additional data or reject the application, which would be a major negative for the shares.
- Legal overhang - A class-action investigation has been reported, which could create headline risk, distraction for management and potential costs.
- Valuation and execution mismatch - EV/Sales ~75x implies substantial revenue growth is expected. If revenue growth disappoints or the company needs more capital, the stock can re-rate sharply lower.
- Cash burn and dilution - Negative free cash flow of about $324.8M and nearly a billion shares outstanding mean dilution or debt raises are possible if revenue doesn't scale fast enough to cover cash needs.
- Short squeezes and elevated volatility - High short interest (135M shares as of 01/30/2026) and recent large short-volume days mean price moves can be violent in either direction, which raises execution risk for both entries and exits.
Counterargument to the bullish thesis
One reasonable counterargument is that the market has already priced in the most likely positive regulatory outcome and current revenue growth. With P/S >70x, any execution misstep or incremental demand weakness will cause a large de-rating. In that view, the upside is limited relative to downside because so much future success is already priced in.
Conclusion and what would change my mind
My view: a tactical mid-term long trade around $6.08 to capture regulatory and revenue-driven upside is appropriate for speculative allocations, provided strict stops are used and position size is limited. The trade depends on continued constructive regulatory dialogue, visible commercial traction and no material negative legal developments.
I would change my view if any of the following occur: an FDA request for large new trials or a formal rejection of ANKTIVA (would be bearish and invalidate this trade), a material miss or guidance cut on revenue (would push me to exit), or a major favorable regulatory milestone such as a formal accept-for-review or expedited review date (would make me consider holding through a longer-term position and potentially raise my target).
Plan your position sizing, set your stop at $4.50, and reassess after each major catalyst. This is a trade on execution - respect the binary nature of biotech outcomes and let headlines dictate adjustments to the plan.
Trade summary: Long IBRX at $6.08, target $8.25, stop $4.50 - mid term (45 trading days) - high risk.